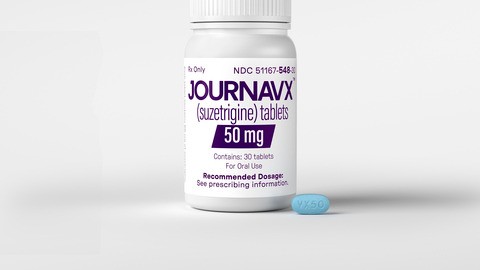
The FDA approved in January 2025 a first of its drug Journavx (generic name: suzetrigine). It is a great innovation in pain management. Opioids are potential for addiction and side effects, but Journavx provides a non-opioid option for managing moderate to severe acute pain. Vertex Pharmaceuticals created this medication.

What Is Suzetrigine (Journavx)?
Suzetrigine is an oral drug that selectively blocks the NaV1.8 sodium channels in peripheral nerves, which are mainly responsible for carrying pain signals from the injury site to the brain. By blocking these channels, Suzetrigine blocks the perception of pain without interfering with the central nervous system, thus eliminating the risk of addiction that comes with opioids.

How Does It Work?
Suzetrigine acts peripherally by stabilizing pain conducting channels within nerve cells. The action makes it possible to give effective analgesia without the potential for euphoria or addiction.

Clinical Efficacy
➔ Suzetrigine in clinical trials showed impressive pain alleviation in individuals subjected to procedures like bunionectomy and abdominoplasty.
➔ Subjects experienced a decrease in pain by 3.5 points on a 0 to 10 scale, similar to the relief achieved through opioid products such as hydrocodone and acetaminophen.

Safety Profile
★ Suzetrigine has a good safety profile.
★ Side effects: mild itching, muscle spasms, and rash.
★ It does not induce nausea, dizziness, or constipation like other alternatives.
★ No evidence of addictive potential has also been seen, which makes it a safer choice for pain relief.
Conclusion
Suzetrigine is a game changer in pain relief. Its effective relief presents a hopeful alternative for patients with moderate to severe acute pain. As the medical profession continues to look for safer pain relief methods, Suzetrigine is a hope.