Casgevy is the first revolutionary gene-editing therapy to be approved for the treatment of beta-thalassemia and severe sickle cell disease in patients aged 12 and older.
Beta thalassemia and sickle cell disease (SCD) are two inherited genetic disorders that affect the production or function of hemoglobin, the protein in red blood cells responsible for oxygen transport throughout the body. Both conditions are chronic, debilitating, and life-threatening.
What is Casgevy
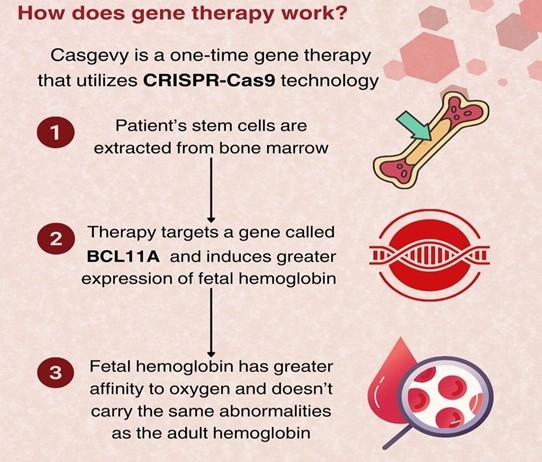
Casgevy is a cell-based gene therapy medicinal product that uses CRISPR/Cas9 technology to edit the patient’s own blood stem cells. It is a personalized one-off treatment that is mainly involved in mobilizing bone marrow stem cells from a patient’s blood.
Working of Casgevy: CRISPR/Cas9 technology
To make Casgevy, stem cells are edited by a technology – CRISPR/Cas9. CRISPR gene-editing finds a specific sequence of DNA inside a cell. It uses ‘molecular scissors’ to make precise cuts, thereby adding, removing or altering genetic material at that specific location of the genome of the cells.
With Casgevy, stem cells are edited at the erythroid-specific enhancer region of the BCL11A gene that usually prevents the production of foetal haemoglobin (HbF). These modified cells are later infused back into the patient, and the reduction of BCL11A gene transcription leads to an increase of HbF production, thereby providing functioning haemoglobin.
The outcome of this genetic modification in long-term hematopoietic stem cells has shown a notable elevation in fetal hemoglobin levels, and also shown a reduction in vaso-occlusive events, thereby forestalling the need for blood transfusions
Implications and Benefits of Casgevy
🩸 Clinical Impact: Reducing the need for blood transfusions and alleviating symptoms thereby showing promising results in clinical trials.
🌍 Global Significance: Holds huge promise for millions of people affected by beta-thalassemia and sickle cell disease worldwide, especially in high-incidence regions.
🔮Future of Medicine: This therapy will set a precedent for future gene editing advancements of other genetic disorders.
Conclusion
CRISPR/Cas9, demonstrated by Casgevy, represents a pivotal advancement in genetic disorder treatment. Casgevy holds the potential for a therapeutic approach, promising recovery for many patients. This revolutionary approach not only prevents the need for recurrent transfusions but promises a drastic improvement in the quality of life for individuals fighting with the multifaceted challenges of SCD and beta-thalassemia. CRISPR/Cas9, with its safety, curative potential, and transformative impact, stands as a flare in the progress of medical science, thereby signifying a paradigm shift in the management of SCD and beta-thalassemia